Safeguarding Toxic & Expensive Drugs
Glass vials are still widely used in laboratories, hospitals or other medical settings. Injection vials are often filled with substances that could potentially harm users and compromise occupational safety, for example, cytotoxic drugs used in chemotherapy. Moreover, many biological drug formulations that are commonly packaged in glass vials are very costly.
For those reasons, it is essential to safeguard the vials against breakage and leakage. Röchling Medical was consulted to develop a protective plastic packaging solution to achieve just that.
Requirements:
- Robust, secure plastic protectors for glass injection vials
- Secure protection against breakage and leakage
- Must pass drop tests at different heights
- Suitable for different vial sizes
- Vial label needs to be readable through the protective packaging
Forward-Thinking Design & Intelligent Tooling
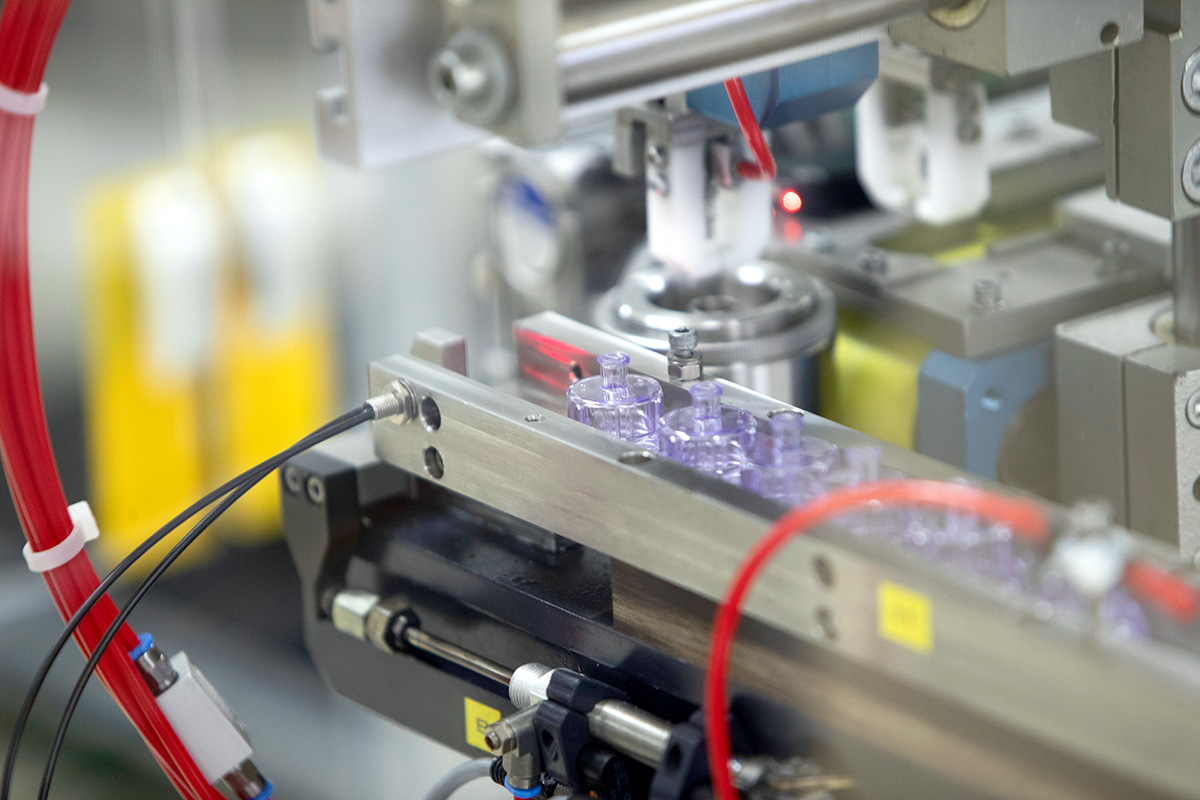
We designed a protective solution that holds each glass vial firmly in place with no rattling or movement, thereby eliminating the risk of breakage or leakage while in transit or during application.
Our standardized outer packaging can accommodate glass vials of different sizes (3 ml to 20 ml), passes all drop tests and is made from a transparent material to allow vial labels to be read.
Our team achieved this by developing a multi-tool, consisting of a fixed tool part for the outer casing of the protective container and exchangeable tool inserts. This allowed for the tool core to be flexibly replaced depending on the required vial size (3 ml to 20 ml).
We applied the same outer geometry, but different inner geometries to accommodate for spacers and centering elements for the range of different vial sizes. In addition, we opted to use snap-on caps instead of screw caps: the simpler closure mechanism ensures easier processing on the filling line.
Smart Standard Solution for the Protection of Various Vial Sizes
Overall, the implementation of the project was geared towards sustainability.
Design for manufacturability principles were systematically applied. This concerns on the one hand the design of the outer packaging in terms of item weight, number of tools and tooling costs and, on the other hand, ease of filling and final assembly on the part of the customer.
Advantages:
- One standard protective solution fits vials from 3 ml up to 20 ml.
- Due to the protective vials' standardized outer geometry, the customer can fill and process all sizes on the same filling line.
- The economic multi-tool set-up with exchangeable tool core dramatically reduces the required number of different tools for manufacturing protective containers for a range of different vial sizes.
- The snap-on cap can be pressed on in a single step after filling and reduces the need for handling on the customer's side.
Vial Protectors


Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our innovative plastic protectors for injection vials, please contact our team.
We look forward to hearing from you.

Thierry Arnaud
Vice President - Sales & Marketing Europe