Integrated Quality Management System
We meet our customers specifications and the regulatory requirements for products and processes on the basis of a certified and integrated management system, under the DIN EN ISO 9001, ISO 13485 and ISO 15378 standards.

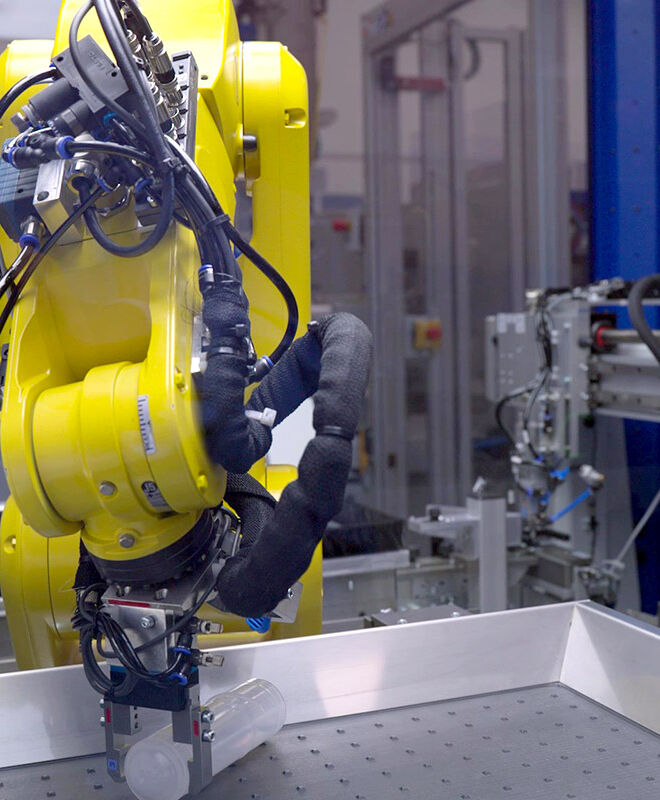
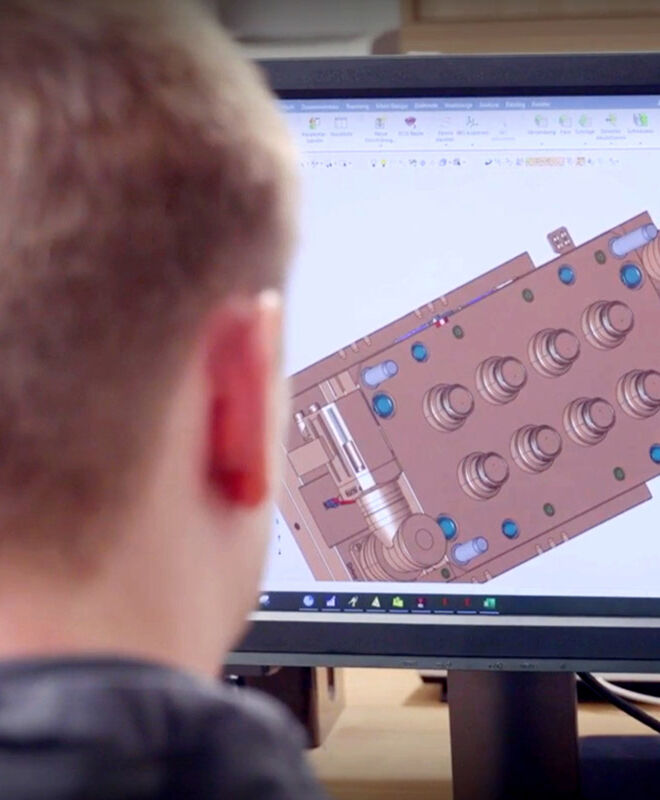
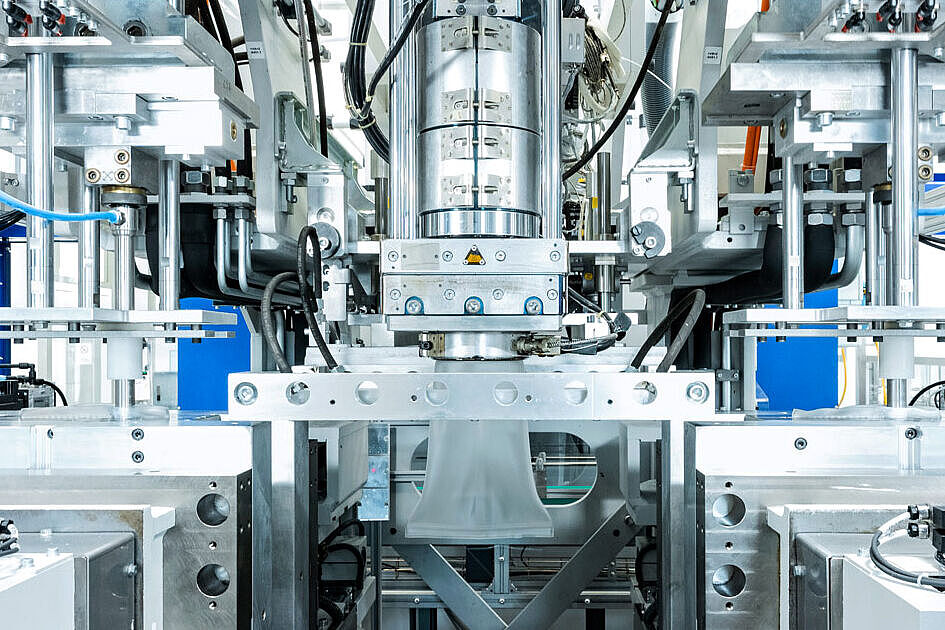
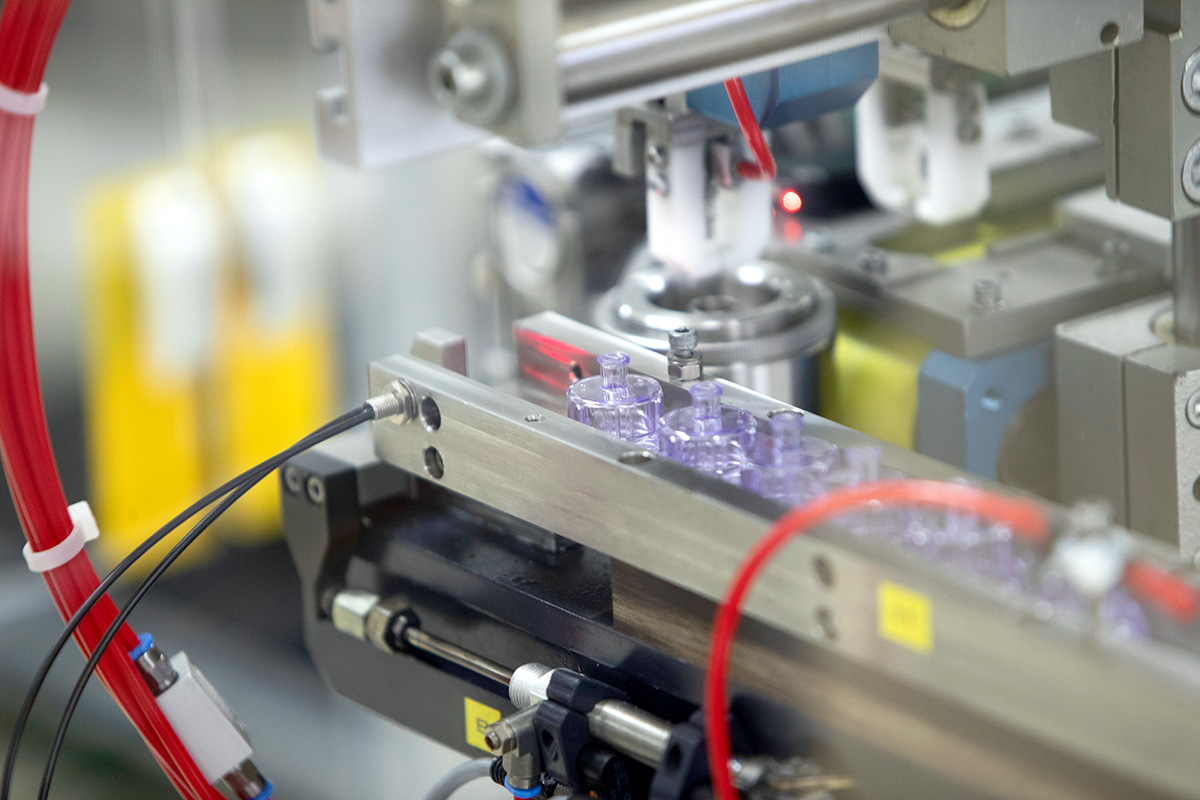
State-of-the-Art, Automated Facilities
Our state-of-the-art facilities are highly automated in all process steps, from manufacturing, assembly and quality inspection to transfer and packaging. We are able to engineer production processes that exclude the risk of contamination and ensure stable processes for consistent, reproducible quality.