

At our plant in Neuhaus, we produce several million containers a year in our GMP C cleanrooms under the strictest controls, extensive hygiene measures and using state-of-the-art technologies.
We have to protect our products from people so carefully that the resulting product and its contents can later protect people.


The small and intensively trained team working in this production area are subject to the strictest clothing regulations: Beard protection, hood, protective overalls, gloves and special shoes.
GMP (Good Manufacturing Practice) is an EU standard for classifying cleanrooms. A GMP C class cleanroom must fulfill the following conditions, among others:
The air must be particularly low in particles. A maximum of 3,520,000 particles with a maximum diameter of 0.5 micrometers (µm) per cubic meter (m³) of air may circulate here, and only 29,000 particles per m³ of air that are larger than 5.0 µm.
By comparison, there can be hundreds of millions of particles in the air in an average cafeteria. Accordingly, the air in the GMP C cleanroom is continuously monitored to ensure compliance with these strict requirements.

Sensor technology is used to monitor not only the number of particles, but also the humidity, temperature, and room pressure. The air pressure in the GMP C cleanroom is higher than in the adjacent GMP D area. This prevents particles from the outside getting inside. To ensure the quality of the plastic packaging from the cleanroom, these conditions must remain constant.
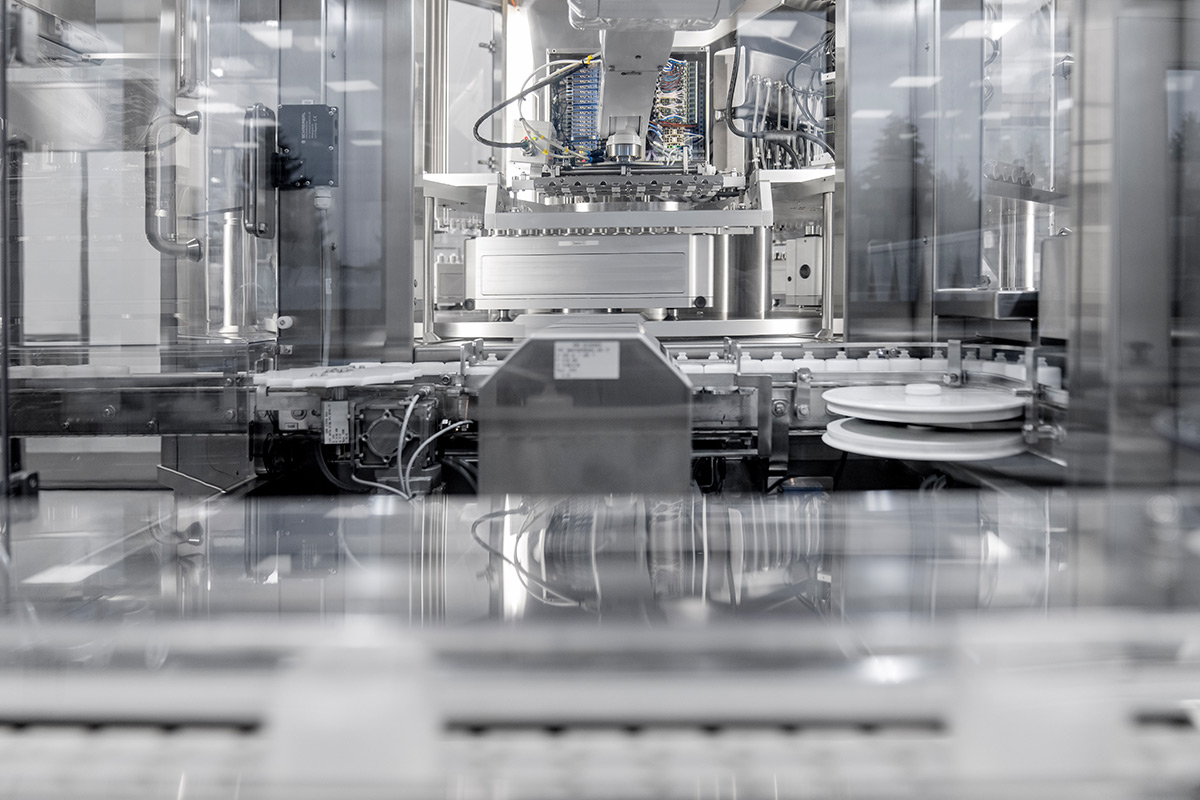
The surfaces must also be specially designed. Stainless steel is used almost entirely here, as this ensures thorough cleaning and maintenance. All types of equipment have been designed to meet this requirement.
The strict incoming inspections apply not only to the employees, but also to the production materials. The plastic granulate for the plastic packaging from the clean room is also fed in at great expense and then transported directly to the machines using a vacuum conveyor system.
15 cameras check the product for various defect characteristics. The containers are transported past the cameras by vacuum and checked at the same time. The smallest defect leads to the immediate ejection of the container. In this way, we can ensure that only good parts leave the quality control.
In the adjoining laboratory of the production area, sample containers are also precisely checked for all defined dimensions, such as weight, collar height or layer thickness. Even the slightest deviation would lead to sorting out.

Once the quality inspection has been successfully completed, the containers are automatically filled into sterile transport bags. Full bags are then sealed and completely sterilized again.
By consistently adhering to these strict hygiene requirements, the use of cutting-edge technology and the strictest quality controls, we ensure that only first-class products leave our factory for further processing and ultimately for patient treatment.


Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical technology and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
For more information about our custom plastic packaging solutions, please contact our team.
We look forward to hearing from you.

Thierry Arnaud
Vice President - Sales & Marketing Europe

Bill Ruth
Vice President - Sales & Marketing