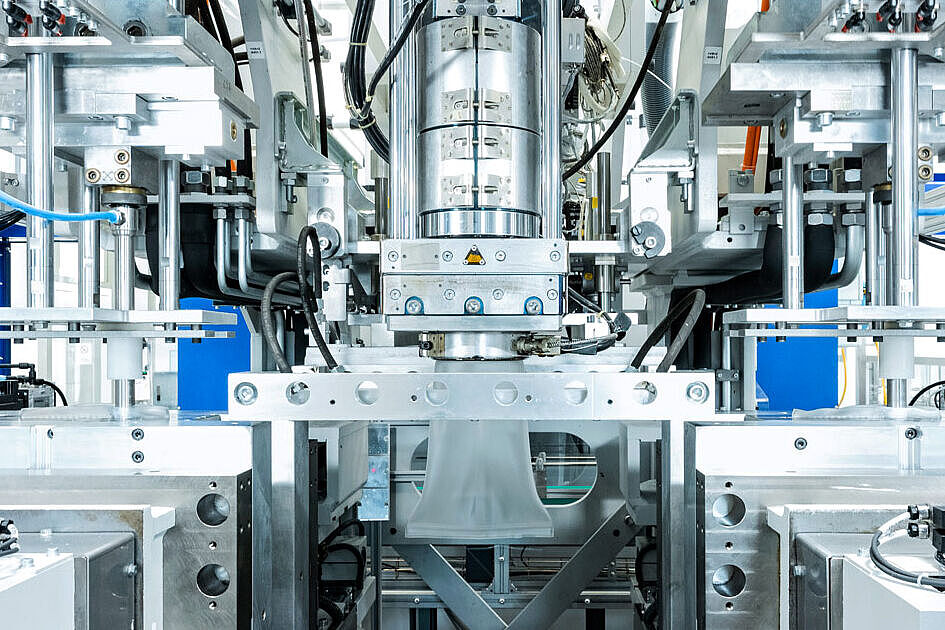
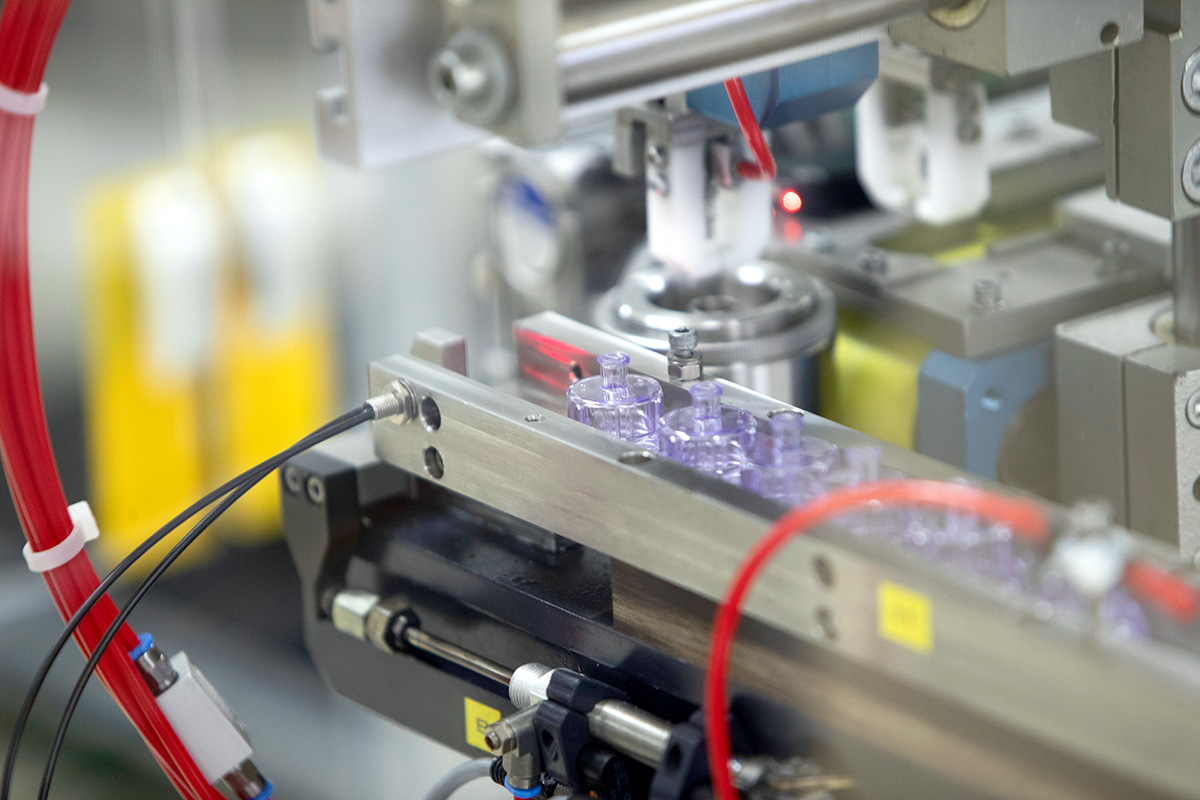
Plastic & Metal Processing Under Certified Cleanroom Conditions
Röchling Medical stands for expertise in cleanroom manufacturing. For the production of high-quality components and assemblies for the medical markets, we offer a wide range of thermoplastic and metal processing technologies in cleanrooms ISO 8 and ISO 7. For the pharma industry, we produce highly pure primary packaging in GMP-certified environments and in ISO 5 cells for aseptic requirements with extremely low particle count.
Our Cleanroom Scope at a Glance
| Certification | Neuhaus Germany | Brensbach Germany | Waldachtal Germany | Rochester USA | Lancaster USA | Suzhou China |
| Cleanroom ISO 8, DIN EN ISO 14644-1 | — | X | X | X | X | X |
| Cleanroom ISO 7, DIN EN ISO 14644-1 | — | — | — | — | X | — |
| Cleanroom ISO 5, DIN EN ISO 14644-1 | X | X | — | — | — | — |
| Cleanroom GMP class D | X | X | — | — | — | — |
| Cleanroom GMP class C | X | X | — | — | — | — |
Processing Technologies |
|
|
|
|
|
|
Microbiological Monitoring & Automated Processes for Superior Hygiene Standards
We meet our pharma customers' demands for highest purity with our inhouse microbiological monitoring capabilities and highly automated facilities.
With our S1 laboratory at our Neuhaus site, we are able to carry out the complete microbiological monitoring of our production environment ourselves, from sampling to evaluation. This allows us to react quickly and preventively to maintain the required high hygiene standards. Fully automated processes optimize our production flows and avoid contamination. Networked systems coordinate the entire process chain and ensure continuous traceability.

"Good Manufacturing Practice" (GMP)

GMP refers to guidelines for quality assurance of the production processes and environment in the production of drugs and active pharmaceutical ingredients. EU GMP regulations include requirements for quality systems, premises and equipment, documentation and traceability.
As suppliers to the pharmaceutical industry, the Röchling Medical sites in Brensbach and Neuhaus are certified according to ISO 15378 and produce strictly according to GMP specifications in cleanrooms of GMP class C + D.
All of our Röchling Medical sites actively live by the principles of GMP. We continuously invest in the expertise and further training of our employees with GMP, safety, hygiene and cleanroom training courses.
Competences at the Following Locations
Case Studies
As a solution provider, we combine our expertise in research & product development, materials & technologies, and automation & industrialization with our experience, our quality standards, and our ambition to work with our customers to design a product that meets their individual requirements.
Our case studies give you an insight into the challenges faced by our customers in pharma, diagnostics and medical technology sectors and the tailor-made solutions that we jointly created with them.

Patient-Centric Pharmaceutical Packaging Design
Optimizing a Complex Class 3 Medical Device

Innovative Plastic Protectors for Glass Injection Vials

Custom Automation Concepts
Design for Manufacturability

Automated Quality Control

Metal and Plastic Processing from a Single Source
Medical Device Contract Manufacturing

A Single Use Medical Device Designed for Sustainability

Simulations in Product Development
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical technology and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our cleanrooms and certifications, please contact our team.
We look forward to hearing from you.

Roman Borkowski
Chief Quality & Innovation Officer