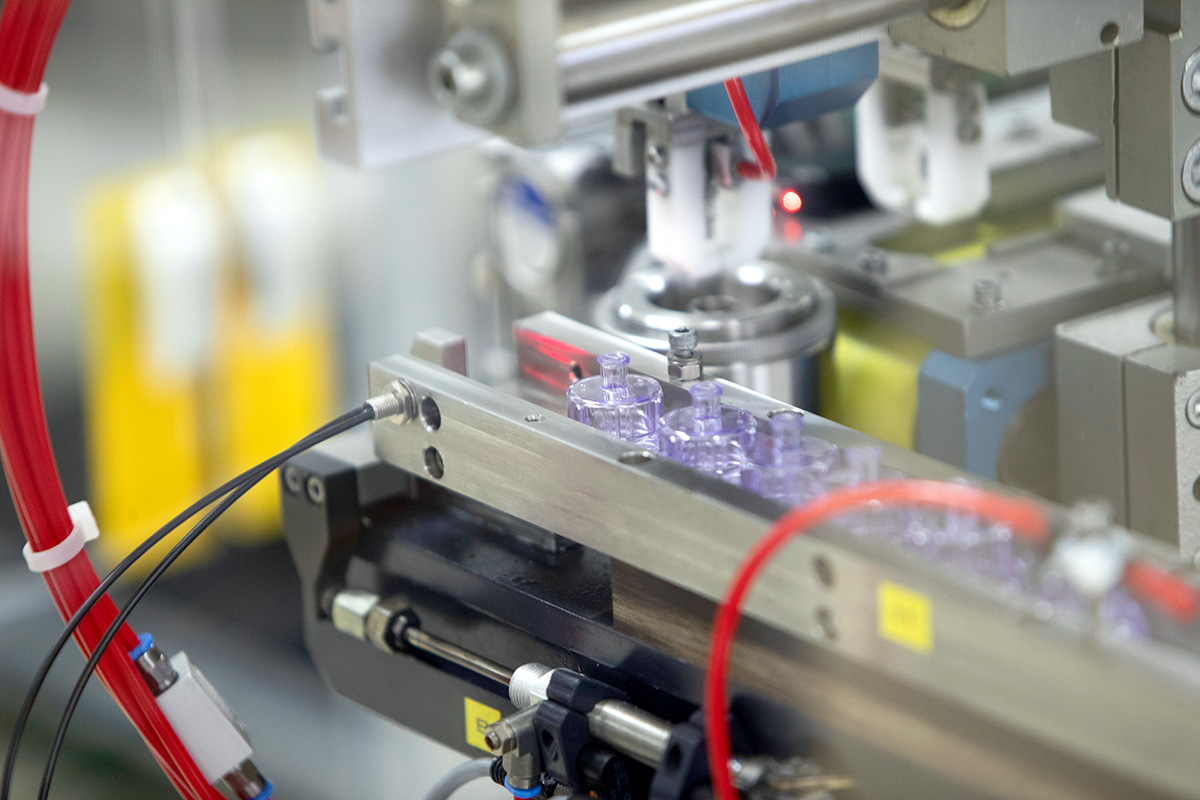
Microbiological Monitoring & Automated Processes for Superior Hygiene Standards
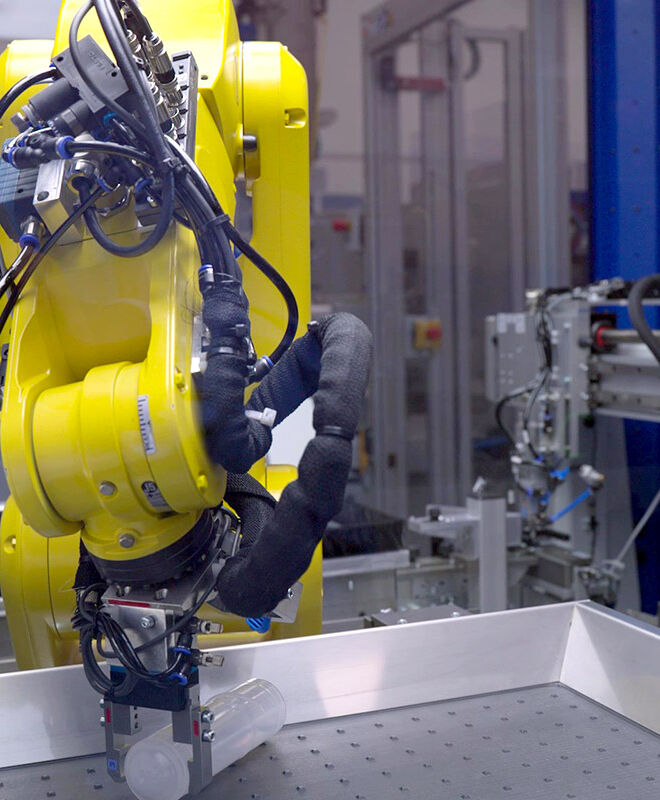
We meet our pharma customers' demands for highest purity with our inhouse microbiological monitoring capabilities and highly automated facilities.
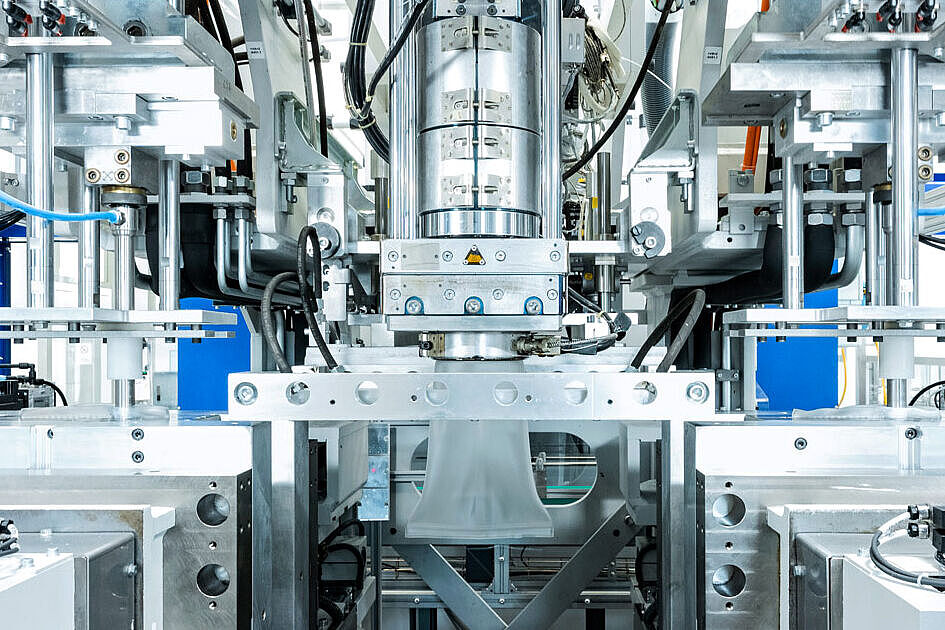
With our S1 laboratory at our Neuhaus site, we are able to carry out the complete microbiological monitoring of our production environment ourselves, from sampling to evaluation. This allows us to react quickly and preventively to maintain the required high hygiene standards. Fully automated processes optimize our production flows and avoid contamination. Networked systems coordinate the entire process chain and ensure continuous traceability.