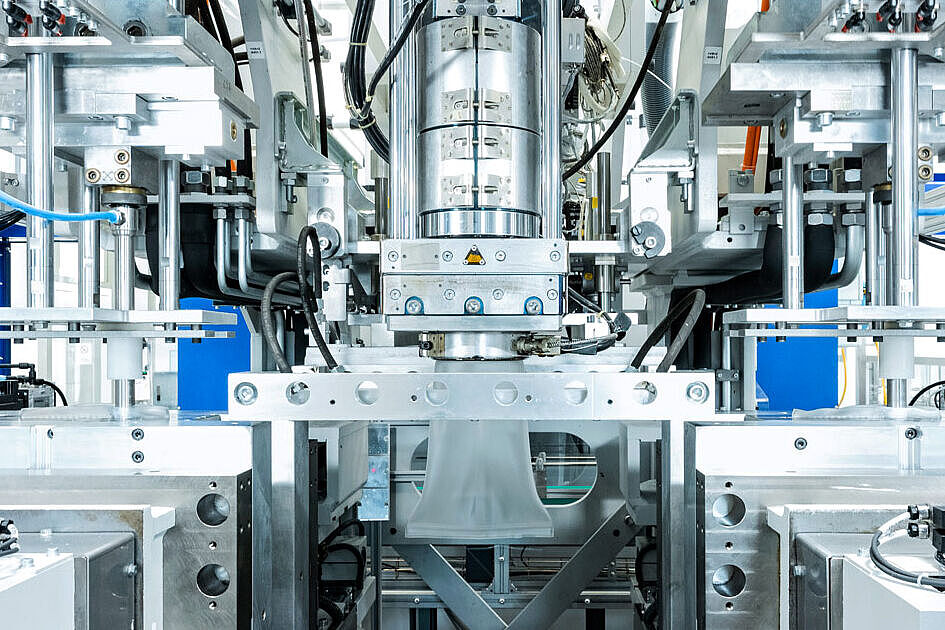
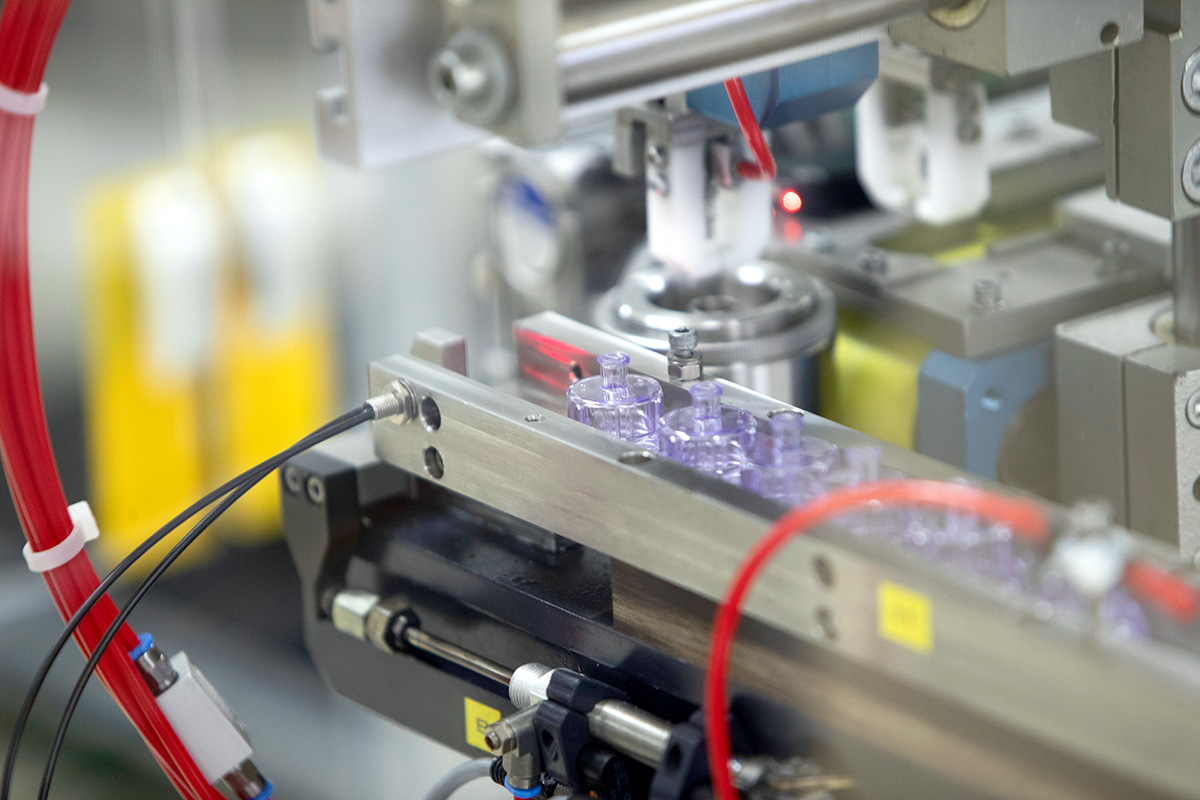
Applications & Areas of Use
AT-Closed Vials® are ready-to-fill vials for injectable drugs, designed to be used in aseptic fill finish processing of biopharmaceutical drugs.
The vials are particularly suitable for use with sensitive advanced therapeutical products such as cell and gene therapy preparations with special requirements for processing, transport and storage.
The polymer injection bottles also offer advantages in the processing and handling of hazardous substances such as cytotoxic oncological drugs or radiopharmaceuticals.
AT-Closed Vials® are designed to be seamlessly fed into robotic filling lines for automated, aseptic filling.

Product Features Overview
The AT-Closed Vials® are injection-molded from clear-as-glass COC (Cycloolefin-Copolymere). The advantages of plastic injection bottles as glass replacement are particularly significant for biological drugs that require transportation, storage, freezing and thawing.
The break-resistant material can withstand the stresses of cryogenic freezing with no loss of container-closure integrity. The polymer vials have a low leachables profile for biopharmaceuticals, avoiding impurities or adverse reactions that could inhibit the efficacy and safety of the contained active substance. The higher break resistance of plastic compared to glass vials avoids the risk of contamination when handling highly potent or cytotoxic drugs.
- Fully automated manufacturing process
- Vials and stoppers produced and assembled under ISO 5 cleanroom conditions
- 100% in-line camera inspection
- Produced in sizes 1 ml and 2 ml
- AT-Closed Vials® are delivered sterile, clean and ready-to-fill


Our Expertise
Automated Two-Component Processing for 100% Purity
The particular challenge in the production of closed ready-to-fill vials is to ensure that the process and the end product are absolutely germ-free. We use a technically complex injection molding tool, which allows both components of the end product to be completed in one production cycle. In a fully automated manufacturing process, the vial and stopper are injection molded and immediately robotically assembled, all under ISO 5 cleanroom conditions. This minimizes particle content compared to conventional manufacturing and assembly processes.
Vial Formats



Cleanroom competence
As specialists in the production of high-standard medical packaging, we know how to design cleanroom concepts meeting the exceptionally high requirements of aseptic manufacturing.

Automation expertise
Our strength is the development of highly automated manufacturing concepts, including robotic assembly and automated in-line controls for the highest quality and purity requirements.

Complex mold design
We have the mold-making competence and material expertise to design complex molds for multi-component injection molding.

Excellent quality systems
Our certified quality management system and tailor-made quality controls ensure consistently excellent product quality meeting the highest requirements for dimensional accuracy and optical flawlessness.
Manufacturing at the Following Locations
Röchling Medical Competences
Our Expertise, Your Benefit
Our customers benefit from our extensive expertise in plastics and metal processing, but also from our many years of experience in medical device and pharma. As your solution partner, we are familiar with both the regulatory and practical requirements of creating components and products for the healthcare sector tailored to your needs. We meet the highest quality and hygiene standards and operate in strict compliance with relevant regulations, such as the Medical Device Regulation (MDR).
Contact Us
For more information about our solutions for pharmaceutical primary packaging and drug delivery, please contact our team. We look forward to hearing from you.

Thierry Arnaud
Vice President - Sales & Marketing Europe